Short Answer
Consider the transformation of A to B (i.e., A →
B). If at equilibrium at 25°C the concentration of A is 20% of the initial concentration of A, determine the value of 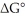
(in kcal/mol) for this reaction. R = 1.987 cal/mol∙K.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q111: Explain the significance of the frequency factor
Q112: Which of the following reactive intermediate species
Q113: Given a ΔG°<sup> </sup>of -8.0 kJ/mol at
Q114: Which of the following species is not
Q115: Energy is _ when bonds are formed
Q117: Consider the one-step conversion of F to
Q118: Write a detailed, stepwise mechanism for the
Q119: What term describes the highest-energy structure in
Q120: Consider the reaction energy diagram shown below.
Q121: Given that tertiary H atoms react with