Essay
Consider the reaction energy diagram shown below. Label the axes.
a. Which points on the curve indicate transition states? Label them with A, B, etc.
b. Is the overall reaction exothermic or endothermic? 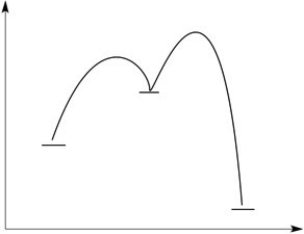
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q115: Energy is _ when bonds are formed
Q116: Consider the transformation of A to B
Q117: Consider the one-step conversion of F to
Q118: Write a detailed, stepwise mechanism for the
Q119: What term describes the highest-energy structure in
Q121: Given that tertiary H atoms react with
Q122: For the compound below, the number of
Q123: The monochlorination of butane with chlorine gas
Q124: Draw the transition state for the hydrogen
Q125: The relative reactivity of the 1°: 2°: