Multiple Choice
Assume the reaction A + B → C + D proceeds to equilibrium. Calculate the equilibrium concentration of D at  given that the starting concentrations of A and B are 2M and that △G° for the reaction is
given that the starting concentrations of A and B are 2M and that △G° for the reaction is 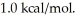
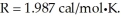
A) 0.40M
B) 0.60M
C) 1.00M
D) 1.40M
E) 1.60M
Correct Answer:

Verified
Correct Answer:
Verified
Q60: The difference in energy between reactants and
Q61: Consider the bond dissociation energies listed below
Q62: Consider the reaction of A being converted
Q63: Which of the halogens below undergoes free
Q64: How many secondary hydrogens are present in
Q66: In the hydrocarbon shown below, how many
Q67: The following reaction occurs readily: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6199/.jpg"
Q68: Draw an energy diagram for a two
Q69: Consider the following substitution reaction with a
Q70: Which of the following correctly expresses the