Short Answer
The following reaction occurs readily: 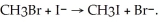
Experimentally one finds that if the concentration of I- is doubled, the rate doubles. Also if the concentration of CH3Br is halved, the rate is halved. What is the rate equation for this reaction?
Correct Answer:

Verified
Correct Answer:
Verified
Q62: Consider the reaction of A being converted
Q63: Which of the halogens below undergoes free
Q64: How many secondary hydrogens are present in
Q65: Assume the reaction A + B →
Q66: In the hydrocarbon shown below, how many
Q68: Draw an energy diagram for a two
Q69: Consider the following substitution reaction with a
Q70: Which of the following correctly expresses the
Q71: When 1,1,3,3-tetramethylcyclobutane is brominated at 125°C, the
Q72: What C<sub>5</sub>H<sub>12</sub> isomer will give only a