Multiple Choice
Which of the following statements correctly describes the contribution of 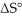 to
to 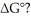
A) The entropy term makes a greater contribution to ΔG° at low temperatures.
B) The entropy term makes a greater contribution to ΔG° at high temperatures.
C) The entropy term makes a greater contribution to ΔG° in exothermic reactions.
D) The entropy term makes a greater contribution to ΔG° in endothermic reactions.
E) The entropy term always makes a more significant contribution to ΔG° than does the enthalpy term.
Correct Answer:

Verified
Correct Answer:
Verified
Q70: Which of the following correctly expresses the
Q71: When 1,1,3,3-tetramethylcyclobutane is brominated at 125°C, the
Q72: What C<sub>5</sub>H<sub>12</sub> isomer will give only a
Q73: Species with unpaired electrons are called _.
Q74: Given the bond dissociation energies below (in
Q76: In the presence of a small amount
Q77: Which compound has the smaller bond dissociation
Q78: Consider the reaction: CH<sub>3</sub>CH<sub>2</sub>∙ + Br<sub>2</sub> →
Q79: The major monobrominated product which results when
Q80: Which of the following is true for