Essay
Consider the reaction: CH3CH2∙ + Br2 → CH3CH2Br + Br∙ .
Given that this reaction has an activation energy of +6 kcal/mol and a 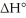
of 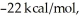
sketch a reaction-energy diagram for this reaction. Label the axes and show Ea and 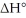
on your drawing.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q73: Species with unpaired electrons are called _.
Q74: Given the bond dissociation energies below (in
Q75: Which of the following statements correctly describes
Q76: In the presence of a small amount
Q77: Which compound has the smaller bond dissociation
Q79: The major monobrominated product which results when
Q80: Which of the following is true for
Q81: Write the structures of all of the
Q82: Will the sign of ΔS° in the
Q83: _ is the study of reaction rates.