Multiple Choice
Consider the reaction (CH3) 3CBr + CH3CH2OH → 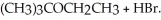 Experimentally one finds that if the concentration of (CH3) 3CBr is tripled, the rate of the reaction triples. One also finds that if the concentration of CH3CH2OH is doubled, the rate of the reaction is unchanged. Which of the following correctly describes the kinetics of this reaction?
Experimentally one finds that if the concentration of (CH3) 3CBr is tripled, the rate of the reaction triples. One also finds that if the concentration of CH3CH2OH is doubled, the rate of the reaction is unchanged. Which of the following correctly describes the kinetics of this reaction?
A) The reaction is third order in (CH3) 3CBr.
B) The reaction is first order in CH3CH2OH.
C) The reaction is second order overall.
D) The reaction is first order overall.
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Provide the structure of the carbene that
Q3: In the reaction of Cl<sub>2</sub> with ethane
Q4: Do you expect the initiation step in
Q5: What is meant by the mechanism of
Q6: The Arrhenius equation mathematically models which of
Q7: Butylated hydroxytoluene, or BHT, is a potent
Q8: How many distinct monochlorinated products can result
Q9: When two carbenes collide, they immediately dimerize
Q10: The rate of a reaction typically increases
Q11: The bond dissociation energy is the amount