Essay
Butylated hydroxytoluene, or BHT, is a potent radical scavenger that is used as a food preservative. It reacts preferentially with free radicals in food to form a new, less reactive resonance stabilized species. The reaction of BHT with a damaging free radical is shown below. Draw two additional resonance structures for the radical form of BHT where the free electron is delocalized. 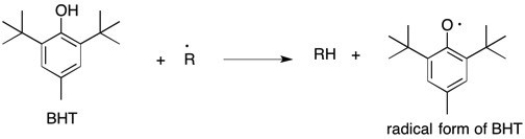
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Consider the reaction (CH<sub>3</sub>)<sub>3</sub>CBr + CH<sub>3</sub>CH<sub>2</sub>OH →
Q3: In the reaction of Cl<sub>2</sub> with ethane
Q4: Do you expect the initiation step in
Q5: What is meant by the mechanism of
Q6: The Arrhenius equation mathematically models which of
Q8: How many distinct monochlorinated products can result
Q9: When two carbenes collide, they immediately dimerize
Q10: The rate of a reaction typically increases
Q11: The bond dissociation energy is the amount
Q12: In the first propagation step of the