Essay
Consider the conversion of X to Z through the sole intermediate Y. Given the reaction-energy diagram shown below, which step is the rate-limiting step? Explain your reasoning. 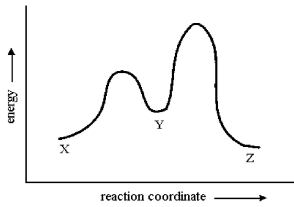
Correct Answer:

Verified
The conversion of Y to Z has a...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q47: Which of the presented mechanisms would be
Q48: Provide the major organic product that results
Q49: Chlorination of methane can result in a
Q50: In the reaction of Cl<sub>2</sub> with ethane
Q51: If the percentage of the axial onformer
Q53: In an exothermic reaction, are stronger bonds
Q54: Which of the following is a propagation
Q55: When acetaldehyde (CH<sub>3</sub>CHO) is deprotonated, the resulting
Q56: How many distinct monochlorinated products can result
Q57: When light is shined on a mixture