Essay
Given an activation energy of 15 kcal/mol, use the Arrhenius equation to estimate how much faster the reaction will occur if the temperature is increased from 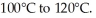
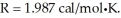
Correct Answer:

Verified
The reacti...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q41: If the equilibrium constant (K<sub>eq</sub>) of a
Q42: The hydrogen atom abstraction step in the
Q43: The hydrogen atom abstraction step in the
Q44: What reactive intermediate is formed when CHBr<sub>3
Q45: Provide the structure of the transition state
Q47: Which of the presented mechanisms would be
Q48: Provide the major organic product that results
Q49: Chlorination of methane can result in a
Q50: In the reaction of Cl<sub>2</sub> with ethane
Q51: If the percentage of the axial onformer