Essay
The hybridization of the nitrogen in the molecule shown below is sp2. Briefly explain why. 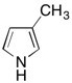
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: What intermolecular attractions exist in a pure
Q2: Which molecule below is an alkene?<br>A) CH<sub>3</sub>CH<sub>2</sub>OCH<sub>2</sub>CH<sub>3</sub><br>B)
Q4: Use the following structure for the two
Q5: What kind of molecular orbital (σ, σ<sup>*</sup>,
Q6: The HNC bond angle in the cation
Q7: Which of the following statements about π
Q8: Sodium hydride (NaH) is a base that
Q9: Which of the following statements concerning the
Q10: An orbital can be described by its
Q11: The HCH bond angle in allene (H<sub>2</sub>CCCH<sub>2</sub>)