Multiple Choice
Which of the following statements concerning the cyclic molecule shown is not true? 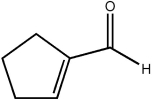
A) It contains a π molecular orbital formed by the overlap of a carbon p atomic orbital with an oxygen p atomic orbital.
B) It contains a σ molecular orbital formed by the overlap of two carbon sp2 hybrid atomic orbitals.
C) It contains a σ molecular orbital formed by the overlap of two carbon sp3 hybrid atomic orbitals.
D) It contains a π molecular orbital formed by the overlap of two carbon p atomic orbitals.
E) It contains a σ molecular orbital formed by the overlap of a carbon p atomic orbital with an oxygen sp3 atomic orbital.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Use the following structure for the two
Q5: What kind of molecular orbital (σ, σ<sup>*</sup>,
Q6: The HNC bond angle in the cation
Q7: Which of the following statements about π
Q8: Sodium hydride (NaH) is a base that
Q10: An orbital can be described by its
Q11: The HCH bond angle in allene (H<sub>2</sub>CCCH<sub>2</sub>)
Q12: Choose the correct hybridization for the atom
Q13: Consider 1,2-dibromoethene, shown below. Use arrows to
Q14: How many carbon-carbon σ bonds are present