Essay
Consider 1,2-dibromoethene, shown below. Use arrows to represent the individual bond dipoles. Would you expect this molecule to be polar? Briefly explain your reasoning. 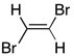
Correct Answer:

Verified
 No, you would not expect this...
No, you would not expect this...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q8: Sodium hydride (NaH) is a base that
Q9: Which of the following statements concerning the
Q10: An orbital can be described by its
Q11: The HCH bond angle in allene (H<sub>2</sub>CCCH<sub>2</sub>)
Q12: Choose the correct hybridization for the atom
Q14: How many carbon-carbon σ bonds are present
Q15: Clonazapam (TM) is used to treat seizures
Q16: Draw a three-dimensional model of methanol, CH<sub>3</sub>OH,
Q17: Choose the correct hybridization for the atom
Q18: From a molecular orbital perspective why isn't