Multiple Choice
The formal charge on the oxygens in the compound below are ________. 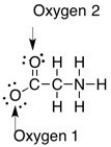
A) Oxygen 1: 0, Oxygen 2: 0
B) Oxygen 1: -1, Oxygen 2: 0
C) Oxygen 1: 0, Oxygen 2: -1
D) Oxygen 1: +1, Oxygen 2: 0
E) Oxygen 1: -1, Oxygen 2: -1
Correct Answer:

Verified
Correct Answer:
Verified
Q98: Complete the following acid/base reaction and use
Q99: Draw additional resonance contributors for: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6199/.jpg"
Q100: How many carbon atoms are present in
Q101: Draw condensed structures for the four compounds
Q102: Rank the following compounds in order of
Q104: The _ tells us that each orbital
Q105: Add the appropriate formal charge to each
Q106: In the compound sodium methoxide (NaOCH<sub>3</sub>), there
Q107: Which of the following condensed formulas correctly
Q108: Assign the correct formal charge to each