Essay
Assign the correct formal charge to each nitrogen atom in the following Lewis structure. (All non-bonding electrons are included.) 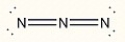
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q103: The formal charge on the oxygens in
Q104: The _ tells us that each orbital
Q105: Add the appropriate formal charge to each
Q106: In the compound sodium methoxide (NaOCH<sub>3</sub>), there
Q107: Which of the following condensed formulas correctly
Q109: Use the curved arrow formalism to indicate
Q110: While you were up late one night
Q111: Provide the line-angle formula (skeletal structure) for
Q112: Draw the important resonance forms for the
Q113: The electronegativity of elements on the periodic