Essay
In the following reaction,identify the Lewis acid and the Lewis base. 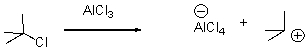
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Which side will the following acid-base reaction
Q3: What is the difference between Ka and
Q4: Can this base exist in liquid ammonia?
Q5: Could water protonate the following compound? HOSO<sub>3</sub><sup>-</sup><br>A)Yes<br>B)No
Q6: Draw arrows to indicate the movement of
Q7: What is the counterion of the t-butyl
Q8: Provide the correct K<sub>eq</sub> for the following
Q9: What is wrong with the following arrow?
Q10: Is the indicated compound acting an acid
Q11: Into which of the following categories does