Multiple Choice
A bottle in a chemical stockroom was labeled simply "dichlorobenzene" without specifying which isomer was present.Capillary GC showed that it was a single pure compound,and the proton decoupled carbon NMR spectrum showed three peaks (not including solvent) .You conclude that the bottle contained
A) 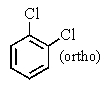
B) 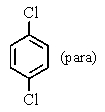
C) 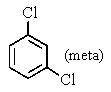
D) any of the dichlorobenzene isomers
E) none of the dichlorobenzene isomers
Correct Answer:

Verified
Correct Answer:
Verified
Q20: Consider the <sup>1</sup>H-NMR of the molecule shown
Q21: The CH<sub>2</sub> group indicated would most likely
Q22: A chemist is evaluating a proton NMR
Q23: A compound of the formula C<sub>10</sub>H<sub>14</sub> gave
Q24: Which of the following is not true
Q26: The most downfield proton NMR signal (i.e.,signal
Q27: The following spectra data was most likely
Q28: A very old bottle labeled only "chlorinated
Q29: Carbon-13 NMR is<br>A) impossible since <sup>13</sup>C has
Q30: Heating the 4-methylbenzenesulfonate ester of the isomer