Heating the 4-Methylbenzenesulfonate Ester of the Isomer Shown Below in 2,2,2-Trifluoroethanol
Multiple Choice
Heating the 4-methylbenzenesulfonate ester of the isomer shown below in 2,2,2-trifluoroethanol (a highly ionizing solvent of low nucleophilicity) leads two products with the molecular formula C10H18.The major product displays 9 different signals in its 13C NMR spectrum.Two of them occur at relatively low field,about = 120 and 145 ppm,respectively.The 1H NMR spectrum exhibits a multiplet near = 5 ppm (1 H) ; all other signals are upfield of = 3 ppm.Identify the compound. 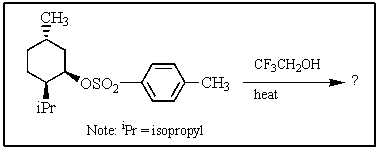
A) 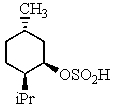
B) 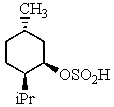
C) 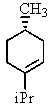
D) 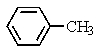
E) 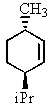
Correct Answer:

Verified
Correct Answer:
Verified
Q25: A bottle in a chemical stockroom was
Q26: The most downfield proton NMR signal (i.e.,signal
Q27: The following spectra data was most likely
Q28: A very old bottle labeled only "chlorinated
Q29: Carbon-13 NMR is<br>A) impossible since <sup>13</sup>C has
Q31: Which compound most likely exhibits the following
Q32: The type of electromagnetic energy required to
Q33: Which of the following structures of formula
Q34: What structure would be consistent with the
Q35: Describe the splitting that would be observed