Multiple Choice
A set of five possible wave functions is given below, where L is a positive real number.
Ψ1(x) = Ae-x, for all x ψ2(x) = A cos x, for all x
Ψ3(x) = 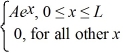
Ψ4(x) = 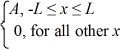
Ψ5(x) = 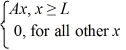
Which of the five possible wave functions are normalizable? (There may be more than one correct choice.)
A) ψ1(x)
B) ψ2(x)
C) ψ3(x)
D) ψ4(x)
E) ψ5(x)
Correct Answer:

Verified
Correct Answer:
Verified
Q9: The wave function for an electron that
Q10: The wave function for an electron that
Q11: A nonrelativistic electron is confined to a
Q13: A molecule of roughly spherical shape has
Q14: A measurement of an electron's speed is
Q15: A nonrelativistic proton is confined to a
Q17: An electron inside a hydrogen atom is
Q19: If the accuracy in measuring the position
Q37: If the accuracy in measuring the velocity
Q39: The square of the wave function of