Essay
The wave function for an electron that is confined to x ≥ 0 nm is
ψ(x) = 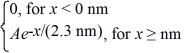
(a) What must be the value of A?
(b) What is the probability of finding the electron in the interval 1.15 nm ≤ x ≤ 1.84 nm?
Correct Answer:

Verified
(a) 0.93 (...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
(a) 0.93 (...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q4: The probability density for an electron that
Q5: A particle is confined to a one-dimensional
Q6: A particle is confined to a one-dimensional
Q10: The wave function for an electron that
Q11: A nonrelativistic electron is confined to a
Q12: A set of five possible wave functions
Q13: A molecule of roughly spherical shape has
Q14: A measurement of an electron's speed is
Q17: An electron inside a hydrogen atom is
Q39: The square of the wave function of