Short Answer
A vertical tube that is closed at the upper end and open at the lower end contains an air pocket. The open end of the tube is under the water of a lake, as shown in the figure. When the lower end of the tube is just under the surface of the lake, where the temperature is 37°C and the pressure is 1.0 × 105 Pa, the air pocket occupies a volume of 630 cm3. Suppose now that the lower end of the tube is at a depth of 86 m in the lake, where the temperature is 7.0°C. What is the volume of the air pocket under these conditions? The density of the water in the lake is 1000 kg/m3. 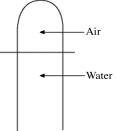
Correct Answer:

Verified
Correct Answer:
Verified
Q7: If a certain sample of an ideal
Q8: How many moles of water (H<sub>2</sub>O)molecules are
Q10: A sealed 26- m<sup>3</sup> tank is filled
Q10: (a)Internal human body temperature is often stated
Q11: Which contains more moles of material: 80
Q12: For a fixed amount of gas,if the
Q17: The figure shows a pV diagram for
Q22: A fixed amount of ideal gas is
Q26: A 25-L container holds ideal hydrogen (H<sub>2</sub>)gas
Q46: An ideal gas is at a pressure