Essay
The condensed structure for dimethyl ether looks symmetrical.However,dimethyl ether has a dipole moment.Draw a structure that explains this and indicate the expected direction of the molecular dipole moment. 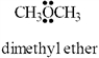
Correct Answer:

Verified
Correct Answer:
Verified
Q40: How many resonance forms can be drawn
Q41: Exhibit 2-2<br>Calculate the formal charges on the
Q42: Exhibit 2-6<br>Refer to the following equation to
Q43: Exhibit 2-1<br>Give the corresponding letter of the
Q44: Exhibit 2-1<br>Give the corresponding letter of the
Q45: Exhibit 2-2<br>Calculate the formal charges on the
Q46: The structure for Vitamin K which is
Q47: Which of the following does not characterize
Q49: Exhibit 2-2<br>Calculate the formal charges on the
Q50: Exhibit 2-1<br>Give the corresponding letter of the