Multiple Choice
The figure (not to scale) shows a pV diagram for 1.8 g of helium gas (He) that undergoes the process 1 → 2 → 3.Find the value of V3.The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K,and the atomic weight of helium is 4.0 g/mol. 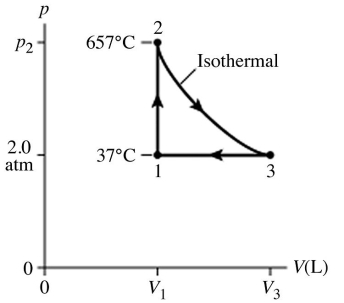
A) 17 L
B) 69 L
C) 34 L
D) 8.6 L
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: A 905-g meteor impacts the earth at
Q34: A substance has a melting point of
Q35: Heat is added to a 2.0 kg
Q36: Some properties of glass are listed here.
Q36: In an isochoric process,the internal (thermal)energy of
Q37: Two metal rods,one silver and the other
Q40: An ideal gas in a balloon is
Q41: A quantity of ideal gas requires 800
Q42: A 648-g empty iron kettle is put
Q46: A steel container,equipped with a piston,contains 21