Multiple Choice
An ideal gas in a balloon is kept in thermal equilibrium with its constant-temperature surroundings.How much work is done by the gas if the outside pressure is slowly reduced,allowing the balloon to expand to 6.0 times its original size? The balloon initially has a pressure of 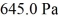 and a volume of
and a volume of 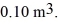 The ideal gas constant is R = 8.314 J/mol ∙ K.
The ideal gas constant is R = 8.314 J/mol ∙ K.
A) 120 J
B) 390 J
C) -330 J
D) 6.0 J
Correct Answer:

Verified
Correct Answer:
Verified
Q7: During an adiabatic process, 20 moles of
Q14: A 905-g meteor impacts the earth at
Q35: Heat is added to a 2.0 kg
Q36: Some properties of glass are listed here.
Q37: Two metal rods,one silver and the other
Q38: The figure (not to scale)shows a pV
Q41: A quantity of ideal gas requires 800
Q42: A 648-g empty iron kettle is put
Q44: The figure shows a pV diagram for
Q46: A steel container,equipped with a piston,contains 21