Short Answer
If a certain sample of an ideal gas has a temperature of 109°C and exerts a pressure of 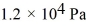 on the walls of its container,how many gas molecules are present in each cubic centimeter of volume? The ideal gas constant is 8.314 J/mol · K and Avogadro's number is
on the walls of its container,how many gas molecules are present in each cubic centimeter of volume? The ideal gas constant is 8.314 J/mol · K and Avogadro's number is
6.022 × 1023 molecules/mol.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: A rod has a length 2.00000 m
Q8: 1)000 L of water at 20.00°C will
Q12: Two steel spheres are made of the
Q20: Sometimes an experiment requires a certain pure
Q21: The figure (not to scale)shows a pV
Q22: The figure shows a pV diagram for
Q26: A 25-L container holds ideal hydrogen (H<sub>2</sub>)gas
Q27: 2.0 L of an ideal nitrogen gas
Q28: The number of molecules in one mole
Q29: The figure shows a pV diagram for