Multiple Choice
A sealed 89- 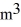 tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K.The gas is heated to a final temperature of 350 K.The ATOMIC mass of oxygen is 16.0 g/mol,and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K.The initial pressure of the gas is closest to
tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K.The gas is heated to a final temperature of 350 K.The ATOMIC mass of oxygen is 16.0 g/mol,and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K.The initial pressure of the gas is closest to
A) 0.15 MPa.
B) 0.17 MPa.
C) 0.19 MPa.
D) 0.13 MPa.
E) 0.11 MPa.
Correct Answer:

Verified
Correct Answer:
Verified
Q10: (a)Internal human body temperature is often stated
Q12: For a fixed amount of gas,if the
Q16: A bag of potato chips contains 2.00
Q35: You want to insert an aluminum rod,which
Q36: The coefficient of volume expansion of olive
Q37: A sealed container holds 0.020 moles of
Q40: What is the mass density of argon
Q41: A certain metal has a coefficient of
Q43: 3.00 moles of an ideal gas at
Q53: When a solid melts,<br>A) the temperature of