Multiple Choice
What is the mass density of argon gas at pressure 1.00 × 105 N/m2 and at temperature 300 K? The mean atomic mass of argon is 39.948 g/mol and the ideal gas constant is R = 8.314 J/mol ∙ K = 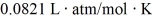 .
.
A) 1.40 kg/m3
B) 1.00 kg/m3
C) 1.20 kg/m3
D) 1.60 kg/m3
E) 1.80 kg/m3
Correct Answer:

Verified
Correct Answer:
Verified
Q12: For a fixed amount of gas,if the
Q30: If the temperature of an iron sphere
Q35: You want to insert an aluminum rod,which
Q36: The coefficient of volume expansion of olive
Q37: A sealed container holds 0.020 moles of
Q38: A sealed 89- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4470/.jpg" alt="A sealed
Q41: A certain metal has a coefficient of
Q43: 3.00 moles of an ideal gas at
Q44: The exterior of a supersonic airplane is
Q53: When a solid melts,<br>A) the temperature of