Multiple Choice
A sealed 26- 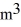 tank is filled with 2000 moles of oxygen gas (O2) at an initial temperature of 270 K.The gas is heated to a final temperature of 460 K.The ATOMIC mass of oxygen is 16.0 g/mol,and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K.The final pressure of the gas is closest to
tank is filled with 2000 moles of oxygen gas (O2) at an initial temperature of 270 K.The gas is heated to a final temperature of 460 K.The ATOMIC mass of oxygen is 16.0 g/mol,and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K.The final pressure of the gas is closest to
A) 0.29 MPa.
B) 0.31 MPa.
C) 0.33 MPa.
D) 0.34 MPa.
E) 0.36 MPa.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: A vertical tube that is closed at
Q10: A cold trap is set up to
Q12: A glass flask has a volume of
Q13: The figure shows a pV diagram for
Q13: The coefficient of linear expansion of aluminum
Q17: A 3.2-L volume of neon gas (Ne)is
Q18: A brass rod is 40.1 cm long
Q19: The figure shows a pV diagram for
Q51: A weather balloon contains 12.0 m<sup>3</sup> of
Q54: A machinist needs to remove a tight