Multiple Choice
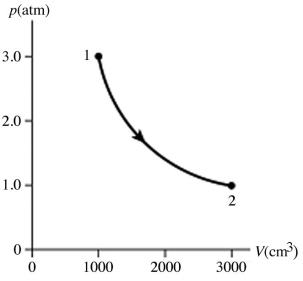
The figure shows a pV diagram for 0.95 mol of gas that undergoes the process 1 → 2.The gas then undergoes an isochoric heating from point 2 until the pressure is restored to the value it had at point 1.What is the final temperature of the gas? The ideal gas constant is R = 8.314 J/mol ∙ K = 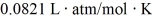 .
. 
A) -160°C
B) 15°C
C) 390°C
D) 120°C
Correct Answer:

Verified
Correct Answer:
Verified
Q12: Two steel spheres are made of the
Q13: The coefficient of linear expansion of aluminum
Q14: A sealed 26- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4470/.jpg" alt="A sealed
Q17: A 3.2-L volume of neon gas (Ne)is
Q18: A brass rod is 40.1 cm long
Q20: Sometimes an experiment requires a certain pure
Q21: The figure (not to scale)shows a pV
Q22: The figure shows a pV diagram for
Q28: The number of molecules in one mole
Q54: A machinist needs to remove a tight