Multiple Choice
Using the bond dissociation energies given,calculate DH° for the following reaction. 
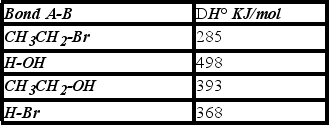
A) +108 KJ/mol
B) -130 KJ/mol
C) -22 KJ/mol
D) +22 KJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: Which of the following statements about a
Q24: The symbol Δ stands for _ in
Q35: Which of the following statements about addition
Q35: Which reaction is slowest? <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7814/.jpg" alt="Which
Q36: Which of the following statements is true?<br>A)Fast
Q40: Which of the following statements about the
Q42: Which of the following expressions summarizes the
Q42: The DG° (free energy change)for the conversion
Q43: Which step would most likely have the
Q45: What type of bond cleavage takes place