Multiple Choice
Which step would most likely have the largest energy of activation? 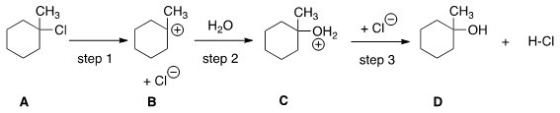
A) Step one
B) Step two
C) Step three
D) It cannot be determined from the information provided
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: Which of the following statements about a
Q24: The symbol Δ stands for _ in
Q35: Which of the following statements about addition
Q40: Using the bond dissociation energies given,calculate DH°
Q40: Which of the following statements about the
Q42: The DG° (free energy change)for the conversion
Q44: Which of the following statements is true?<br>A)The
Q45: What type of bond cleavage takes place
Q48: What kind of reaction does the conversion
Q49: Which of the following statements is true?<br>A)Bond