Multiple Choice
An isotope with a low value of N/Z will generally decay through
A) α decay.
B) β decay.
C) 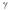 decay.
decay.
D) electron capture.
E) spontaneous fission.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q34: Positron decay and electron capture have the
Q36: Which one of the following elements is
Q52: Iodine-131, t<sub>1/2</sub> = 8.0 days, is used
Q56: A scintillation counter<br>A)measures the signal coming from
Q71: Identify the missing species in the following
Q72: So-called "magic numbers" of particles are thought
Q76: A 9.52 × 10<sup>-5</sup> mol sample of
Q78: The isotope <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7800/.jpg" alt="The isotope
Q79: Select the nuclide that completes the following
Q80: Sodium-21 will emit positrons each having an