Multiple Choice
The isotope 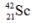 is unstable. This is predictable because
is unstable. This is predictable because
A) the number of neutrons is too large in relation to the number of protons.
B) the number of neutrons is too small in relation to the number of protons.
C) the atomic number is too large.
D) the mass number is too large.
E) Sc isotopes are all unstable.
Correct Answer:

Verified
Correct Answer:
Verified
Q34: Positron decay and electron capture have the
Q36: Which one of the following elements is
Q52: Iodine-131, t<sub>1/2</sub> = 8.0 days, is used
Q56: A scintillation counter<br>A)measures the signal coming from
Q71: Identify the missing species in the following
Q72: So-called "magic numbers" of particles are thought
Q76: A 9.52 × 10<sup>-5</sup> mol sample of
Q77: An isotope with a low value of
Q79: Select the nuclide that completes the following
Q80: Sodium-21 will emit positrons each having an