Multiple Choice
The reaction of methane with water to form carbon dioxide and hydrogen is nonspontaneous at 298 K. At what temperature will this system make the transition from nonspontaneous to spontaneous? The data refer to 298 K. CH4(g) + 2H2O(g)  CO2(g) + 4H2(g)
CO2(g) + 4H2(g) 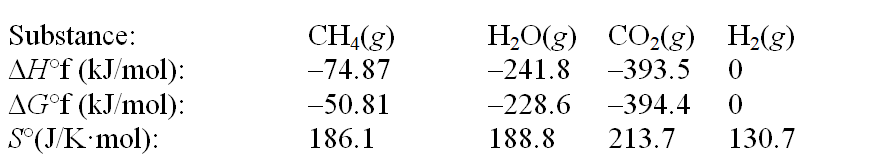
A) 658 K
B) 683 K
C) 955 K
D) 1047 K
E) 1229 K
Correct Answer:

Verified
Correct Answer:
Verified
Q5: A reaction has ΔG = 10.0 kJ
Q6: Elemental boron can be formed by reaction
Q8: Which of the following should have the
Q8: The temperature at which the following process
Q9: Given: H<sub>2</sub>O(l) → H<sub>2</sub>O(s) ΔH° = −6.02
Q11: Calculate ΔG° for the combustion of propane.
Q21: Which relationship or statement best describes ΔS°
Q23: Which relationship or statement best describes ΔS°
Q34: Which relationship or statement best describes ΔS°
Q80: Which relationship or statement best describes ΔS°