Multiple Choice
Which one of the following is the best representation of the titration curve that will be obtained in the titration of a weak base (0.10 mol L-1) with HCl of the same concentration? 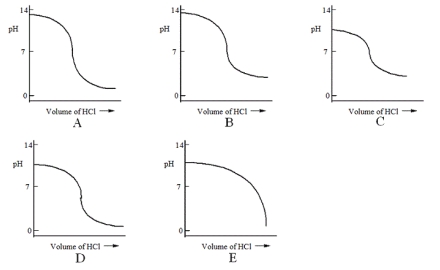
A) A
B) B
C) C
D) D
E) E
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: When a strong acid is titrated with
Q37: If the pH of a buffer solution
Q42: A diprotic acid H<sub>2</sub>A has K<sub>a1</sub> =
Q43: Write the ion product expression for silver
Q44: Which of the following substances has the
Q47: Consider the dissolution of MnS in water
Q48: A solution is prepared by dissolving 20.0
Q49: Buffer solutions with the component concentrations shown
Q50: What is the pH of a solution
Q51: The pH of blood is 7.35. It