Multiple Choice
Write the ion product expression for silver sulfide, Ag2S.
A) 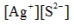
B) 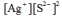
C) 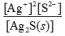
D) 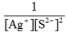
E) 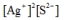
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: When a strong acid is titrated with
Q37: If the pH of a buffer solution
Q38: A solution is prepared by mixing 50.0
Q39: A 20.0-mL sample of 0.30 M HClO
Q40: Which of the following acids should be
Q42: A diprotic acid H<sub>2</sub>A has K<sub>a1</sub> =
Q44: Which of the following substances has the
Q46: Which one of the following is the
Q47: Consider the dissolution of MnS in water
Q48: A solution is prepared by dissolving 20.0