Multiple Choice
When a chemical system is at equilibrium,
A) the concentrations of the reactants are equal to the concentrations of the products.
B) the concentrations of the reactants and products have reached constant values.
C) the forward and reverse reactions have stopped.
D) the reaction quotient, 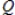 , has reached a maximum.
, has reached a maximum.
E) the reaction quotient, 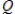 , has reached a minimum.
, has reached a minimum.
Correct Answer:

Verified
Correct Answer:
Verified
Q32: Magnesium carbonate dissociates to magnesium oxide and
Q33: The reaction system POCl<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7800/.jpg" alt="The
Q34: Write the mass-action expression, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7800/.jpg" alt="Write
Q35: Consider the reactions of cadmium with the
Q36: The equilibrium constant, Kp, for the reaction
Q38: The reaction system CS<sub>2</sub>(g) + 4H<sub>2</sub>(g) <img
Q39: At 25°C, the equilibrium constant Kc for
Q40: Nitrogen dioxide decomposes according to the reaction
Q42: Nitric oxide and bromine were allowed to
Q88: In a chemical reaction, if the starting