Multiple Choice
Nitric oxide and bromine were allowed to react in a sealed container. When equilibrium was reached PNO = 0.526 atm, 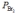 = 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction.
= 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction.
2NO(g) + Br2(g)  2NOBr(g)
2NOBr(g)
A) 7.45 × 10-3
B) 0.109
C) 9.18
D) 91.8
E) 134
Correct Answer:

Verified
Correct Answer:
Verified
Q21: A chemical reaction has an equilibrium constant
Q37: When a chemical system is at equilibrium,<br>A)
Q38: The reaction system CS<sub>2</sub>(g) + 4H<sub>2</sub>(g) <img
Q39: At 25°C, the equilibrium constant Kc for
Q40: Nitrogen dioxide decomposes according to the reaction
Q43: H<sub>2</sub>SO<sub>3</sub>(aq) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7800/.jpg" alt="H<sub>2</sub>SO<sub>3</sub>(aq) HSO<sub>3</sub>(aq)
Q44: Consider the following two equilibria and their
Q45: A mixture of 0.500 mole of carbon
Q47: Which of the following has an effect
Q88: In a chemical reaction, if the starting