Multiple Choice
What is the mass-action expression,  c, for the following chemical reaction? PbO(s) + CO(g)
c, for the following chemical reaction? PbO(s) + CO(g)  Pb(l) + CO2(g)
Pb(l) + CO2(g)
A) 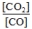
B) 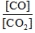
C) 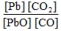
D) 
E) None of these choices are correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Write the mass-action expression, c, for the
Q5: The reaction of nitric oxide to form
Q8: Hydrogen bromide will dissociate into hydrogen and
Q9: Write the mass-action expression, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7800/.jpg" alt="Write
Q10: The equilibrium constant, Kp, for the reaction
Q11: Consider the equilibrium reaction: H<sub>2</sub>(g) + Br<sub>2</sub>(g)
Q16: For a gas-phase equilibrium, a change in
Q53: When a reaction system reaches equilibrium, the
Q63: Once a reaction system reaches equilibrium, the
Q78: If all the reactants and products in