Multiple Choice
The electrochemical reaction that powers a lead-acid storage battery is as follows: Pb(s) + PbO2(s) + 4 H+(aq) + 2 SO42-(aq) → 2 PbSO4(s) + 2 H2O( 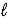 )
)
A single cell of this battery consists of a Pb electrode and a PbO2 electrode,each submerged in sulfuric acid.Which of the following reactions occurs at the cathode during discharge?
A) Pb(s) is reduced to PbSO4(s) .
B) PbO2(s) is reduced to PbSO4(s) .
C) PbO2(s) is oxidized to PbSO4(s) .
D) Pb(s) is oxidized to PbSO4(s) .
E) H+ is oxidized to H2O( 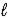 ) .
) .
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Calculate the value of the equilibrium constant
Q10: The value of E°<sub>cell</sub> is <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg"
Q11: Balance the following half-reaction occurring in a
Q12: According to the cell notation below,which of
Q13: Calculate E<sub>cell</sub> for the following electrochemical cell
Q15: Write the balanced oxidation half-reaction for the
Q16: Which of the following statements is true
Q17: Which of the following are the expected
Q18: The unit for electromotive force,emf,is the volt.1
Q19: Which of the following is the cell