Multiple Choice
The value of E°cell is 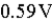 for the following reaction: Cl2(g) + 2 Fe2+(aq) → 2 Fe3+(aq) + 2 Cl-(aq)
for the following reaction: Cl2(g) + 2 Fe2+(aq) → 2 Fe3+(aq) + 2 Cl-(aq)
Calculate the value of E°cell for the reaction below.
Cl-(g) + Fe3+(aq) → Fe2+(aq) + ½ Cl2(g)
A) -1.18 V
B) -0.30 V
C) 0.59 V
D) 0.30 V
E) -0.59 V
Correct Answer:

Verified
Correct Answer:
Verified
Q5: Which of the following statements is true
Q6: The cell potential of the following electrochemical
Q7: One Faraday is defined as the:<br>A) charge
Q8: Which of the following statements is true
Q9: Calculate the value of the equilibrium constant
Q11: Balance the following half-reaction occurring in a
Q12: According to the cell notation below,which of
Q13: Calculate E<sub>cell</sub> for the following electrochemical cell
Q14: The electrochemical reaction that powers a lead-acid
Q15: Write the balanced oxidation half-reaction for the