Multiple Choice
Which of the following overall chemical equations is responsible for generating electricity in fuel cells used in NASA's Space Shuttle programs?
A) Fe2O3(s) + Al(s) → Fe(s) + Al2O3(s)
B) Pb(s) + PbO2(s) + 2 H2SO4(aq) → 2 PbSO4(s) + 2 H2O( 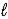 )
)
C) 2 NiO(OH) (s) + Cd(s) + H2O( 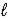 ) → 2 Ni(OH) 2(s) + Cd(OH) 2(s)
) → 2 Ni(OH) 2(s) + Cd(OH) 2(s)
D) 2 H2(g) + O2(g) → 2 H2O( 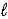 )
)
E) N2H4( 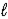 ) + O2(g) → N2(g) + 2 H2O(
) + O2(g) → N2(g) + 2 H2O( 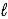 )
)
Correct Answer:

Verified
Correct Answer:
Verified
Q23: Calculate the standard cell potential ( <img
Q24: Primary batteries are also called storage batteries
Q25: For the following cell reaction,the standard cell
Q26: Which of the following is the cell
Q27: Which of the following reactions will require
Q29: The following electrochemical cell has a potential
Q30: In the context of the diagram given
Q31: Which of the following statements concerning voltaic
Q32: When the given oxidation-reduction reaction in an
Q33: Which of the following statements is true