Multiple Choice
For the following cell reaction,the standard cell potential is 1.34 V.To determine the cell potential at nonstandard conditions,what is the value that should be used for n in the Nernst equation? 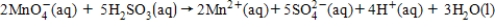
A) 8
B) 10
C) 5
D) 2
E) 6
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: Write a balanced net ionic equation for
Q21: Write a balanced half-reaction for the reduction
Q22: Calculate the equilibrium constant for the following
Q23: Calculate the standard cell potential ( <img
Q24: Primary batteries are also called storage batteries
Q26: Which of the following is the cell
Q27: Which of the following reactions will require
Q28: Which of the following overall chemical equations
Q29: The following electrochemical cell has a potential
Q30: In the context of the diagram given