Multiple Choice
Calculate 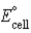 for the electrochemical cell Ag(s) | AgCl(s) | Cl-(aq,1.0 M) || Cu2+(aq,1.0 M) | Cu(s) . The standard reduction potentials are as follows:
for the electrochemical cell Ag(s) | AgCl(s) | Cl-(aq,1.0 M) || Cu2+(aq,1.0 M) | Cu(s) . The standard reduction potentials are as follows:
Cu2+(aq) + 2 e- → Cu(s)
E° = +0.337 V
AgCl(s) + e- → Ag(s) + Cl-(aq)
E° = +0.222 V
A) -0.115 V
B) -0.107 V
C) +0.115 V
D) +0.452 V
E) +0.559 V
Correct Answer:

Verified
Correct Answer:
Verified
Q44: The use of electrical energy to produce
Q45: When a secondary battery provides electrical energy,it
Q46: The standard reduction potentials are as follows:
Q47: Claculate the mass of chromium that can
Q48: Calculate the standard reduction potential for the
Q50: Calculate the charge,in coulombs,is required to deposit
Q51: In an electrolytic cell,reduction occurs at the
Q52: Write balanced reduction and oxidation half-reactions for
Q53: Which of the following is the cell
Q54: Gold and platinum are commonly used as