Short Answer
The use of electrical energy to produce chemical change is known as _____.An example of this process is the reduction of sodium chloride,NaCl( 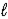 ),to produce solid sodium.
),to produce solid sodium.
Correct Answer:

Verified
Correct Answer:
Verified
Q39: Aluminum(III)ion (Al<sup>3+</sup>)is reduced to solid aluminum at
Q40: Calculate E<sub>cell</sub> for the following electrochemical cell
Q41: Calculate the value of the reaction quotient,Q,for
Q42: The standard cell potential of the given
Q43: Calculate E°<sub>cell</sub> for the cell for the
Q45: When a secondary battery provides electrical energy,it
Q46: The standard reduction potentials are as follows:
Q47: Claculate the mass of chromium that can
Q48: Calculate the standard reduction potential for the
Q49: Calculate <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="Calculate for