Multiple Choice
Calculate the value of the reaction quotient,Q,for the voltaic cell constructed from the following two half-reactions when the Zn2+ion concentration is 0.0110 M and the Ag+ ion concentration is 1.27 M? 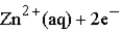 →
→ 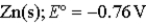
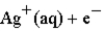 →
→ 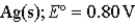
A) 8.66 × 10-3
B) 6.82 × 10-3
C) 115
D) 1.25 × 10-2
E) 147
Correct Answer:

Verified
Correct Answer:
Verified
Q36: For the electrochemical cell Cu(s)| Cu<sup>2+</sup> ||
Q37: The following has a potential of 0.34
Q38: Write a balanced half-reaction for the reduction
Q39: Aluminum(III)ion (Al<sup>3+</sup>)is reduced to solid aluminum at
Q40: Calculate E<sub>cell</sub> for the following electrochemical cell
Q42: The standard cell potential of the given
Q43: Calculate E°<sub>cell</sub> for the cell for the
Q44: The use of electrical energy to produce
Q45: When a secondary battery provides electrical energy,it
Q46: The standard reduction potentials are as follows: