Multiple Choice
Batteries used in watches contain mercury(II) oxide.As the current flows,mercury(II) oxide is reduced to mercury according to the following reaction: HgO(s) + H2O( 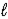 ) + 2 e- → Hg(
) + 2 e- → Hg( 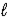 ) + 2 OH-(aq)
) + 2 OH-(aq)
If 2.3 × 10-5 amperes flows continuously for 1200 days,calculate the mass of mercury,Hg( 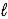 ) ,produced.
) ,produced.
A) 2.5 g
B) 5.0 g
C) 9.9 g
D) 13 g
E) 15 g
Correct Answer:

Verified
Correct Answer:
Verified
Q54: Gold and platinum are commonly used as
Q55: If the <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="If the
Q56: A current of 12.0 A is passed
Q57: Which of the following species are likely
Q58: Explain the function of a salt bridge
Q60: Calculate the copper(II)ion concentration at 25 °C
Q61: Write a balanced chemical equation for the
Q62: If Δ<sub>r</sub>G° for the following reaction is
Q63: How many moles of electrons are produced
Q64: Calculate the equilibrium constant for the reaction