Multiple Choice
If ΔrG° for the following reaction is -22.2 kJ/mol-rxn,calculate 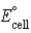 for the following reaction: Cu2+(aq) + 2 Ag(s) + 2 Cl-(aq) → Cu(s) + 2 AgCl(s)
for the following reaction: Cu2+(aq) + 2 Ag(s) + 2 Cl-(aq) → Cu(s) + 2 AgCl(s)
A) -0.460 V
B) -0.115 V
C) +0.115 V
D) +0.230 V
E) +0.559 V
Correct Answer:

Verified
Correct Answer:
Verified
Q57: Which of the following species are likely
Q58: Explain the function of a salt bridge
Q59: Batteries used in watches contain mercury(II)oxide.As the
Q60: Calculate the copper(II)ion concentration at 25 °C
Q61: Write a balanced chemical equation for the
Q63: How many moles of electrons are produced
Q64: Calculate the equilibrium constant for the reaction
Q65: If an electric current is passed through
Q66: Consider the following half-reactions. Ag<sup>+</sup>(aq)+ e<sup>-</sup> →
Q67: Which of the following statements is true