Multiple Choice
Which of the following represents the change in entropy for a system going from 142 possible microstates to 830 possible microstates? (k = 1.381 × 10-23 J/K)
A) 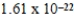 J/K
J/K
B) 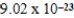 J/K
J/K
C) − 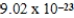 J/K
J/K
D) 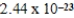 J/K
J/K
E) − 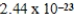 J/K
J/K
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: The standard free energy of formation of
Q3: At what temperatures will a reaction be
Q4: Which of the following linear chain alcohols
Q5: What is the equilibrium constant for the
Q6: Thermodynamics can be used to determine all
Q7: Calculate the enthalpy of vaporization of water
Q8: Which of the following is the first
Q9: What is the sign of ΔH (system)and
Q10: Using the given data,determine ΔrG° at 298
Q11: Calculate Δ<sub>r</sub>G° at 25.0 °C for the