Multiple Choice
An impure sample of sodium carbonate,Na2CO3,is titrated with 0.113 M HCl according to the reaction below. 2 HCl(aq) + Na2CO3(aq) 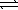 CO2(g) + H2O(
CO2(g) + H2O( 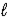 ) + 2 NaCl(aq)
) + 2 NaCl(aq)
What is the percent of Na2CO3 in a 0.613 g sample if the titration requires 26.14 mL of HCl? The molar mass of Na2CO3 is 106.0 g/mol.
A) 0.295%
B) 15.7%
C) 25.5%
D) 51.1%
E) 67.9%
Correct Answer:

Verified
Correct Answer:
Verified
Q38: Hyperventilation can cause your blood pH to
Q39: What is the pH of an aqueous
Q40: A 1.0-liter solution contains 0.25 M HF
Q41: Consider the reaction Cu<sup>2+</sup>(aq)+ 4 NH<sub>3</sub>(aq) <img
Q42: A solution containing 10.mmol of CO<sub>3</sub><sup>2</sup><sup>−</sup>and 5.0
Q44: A 25.00-mL sample of propionic acid,HC<sub>3</sub>H<sub>5</sub>O<sub>2</sub>,of unknown
Q45: To make a buffer with a pH
Q46: What is the pH of a saturated
Q47: The concentration of Pb<sup>2+</sup> in an aqueous
Q48: What is the pH of the buffer